WestVac BioPharma Co., Ltd. has achieved significant progress in the second generation COVID-19 vaccine against the Omicron mutant strain.
WestVac BioPharma Co., Ltd. has achieved significant progress in the second generation COVID-19 vaccine against the Omicron mutant strain.
The vaccine belongs to the latest fifth-generation vaccine technology, targeting the S-RBD protein of the COVID-19 mutant strain, the trimer subunit vaccine antigen is precisely designed based on the structure. The large-scale production of vaccines adopts internationally advanced insect cell production technology and coordinates with international novel vaccine adjuvants.
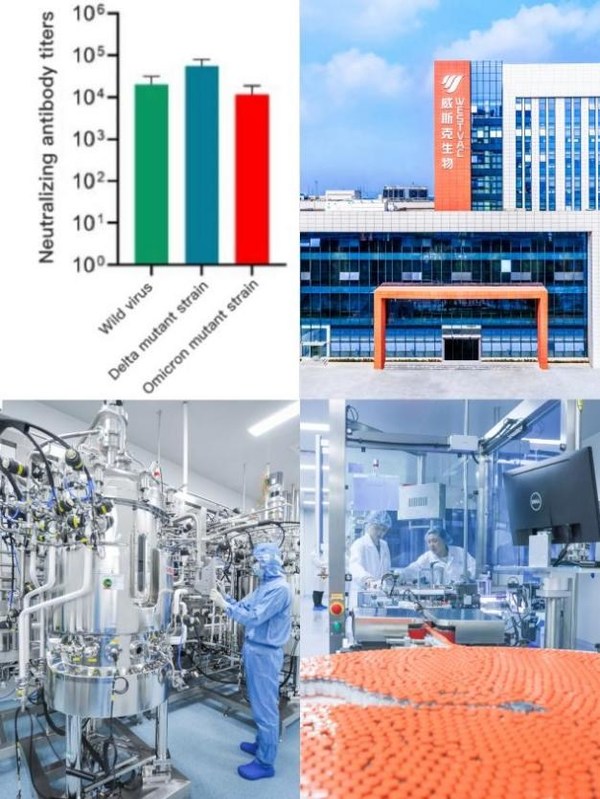
The vaccine can induce the production of high titers of neutralizing antibodies upon immunizing animals such as mice or monkeys. The neutralizing antibodies against the Omicron variant strain can reach a level of ten thousand (when blood is diluted 10,000 times, it still has the ability to block the virus from infecting cells). It was previously discovered that the vaccine has a level of ten thousand neutralizing antibodies that completely inhibits Brazilian strains, South African strains, Delta strains and other mutant strains of Euvirus, suggesting that the vaccine is a universal COVID-19 vaccine against multiple mutant strains, and it is also the first COVID-19 vaccine announced internationally that has high titers of neutralizing antibodies against Omicron. Animal experiments conducted with sequential vaccination of different types of COVID-19 vaccines showed that two doses of mRNA/inactivated vaccine or one dose of adenovirus vaccine, followed by the vaccine, rapidly activated the immune response and produced higher protective neutralizing antibodies than the same type of vaccine.
The vaccine has completed process research and large-scale preparation. The production process is stable and it has been certified by the National Institutes for Food and Drug Control; the pharmacodynamic research on the immunogenicity and protection of mice, rats, and monkeys, as well as pre-clinical safety evaluation, has been completed; it is expected to enter clinical trials in early 2022.
WestVac Biopharma is an international leading vaccine and immunotherapy platform. It is listed as a unicorn company in 2021. WestVac has GMP production workshops with an annual production of 600 million doses and obtained the “Drug Production License” issued by the local medical products administration. The company has mature insect cell expression, mRNA vaccine, novel adjuvant, bacterial vaccine and tumour vaccine and immunotherapy platforms, and has 21 pipelines.
A nasal spray recombinant COVID-19 vaccine against variants is also being developed. Its convenience will boost vaccination worldwide.
Edited by: Stephen Morton